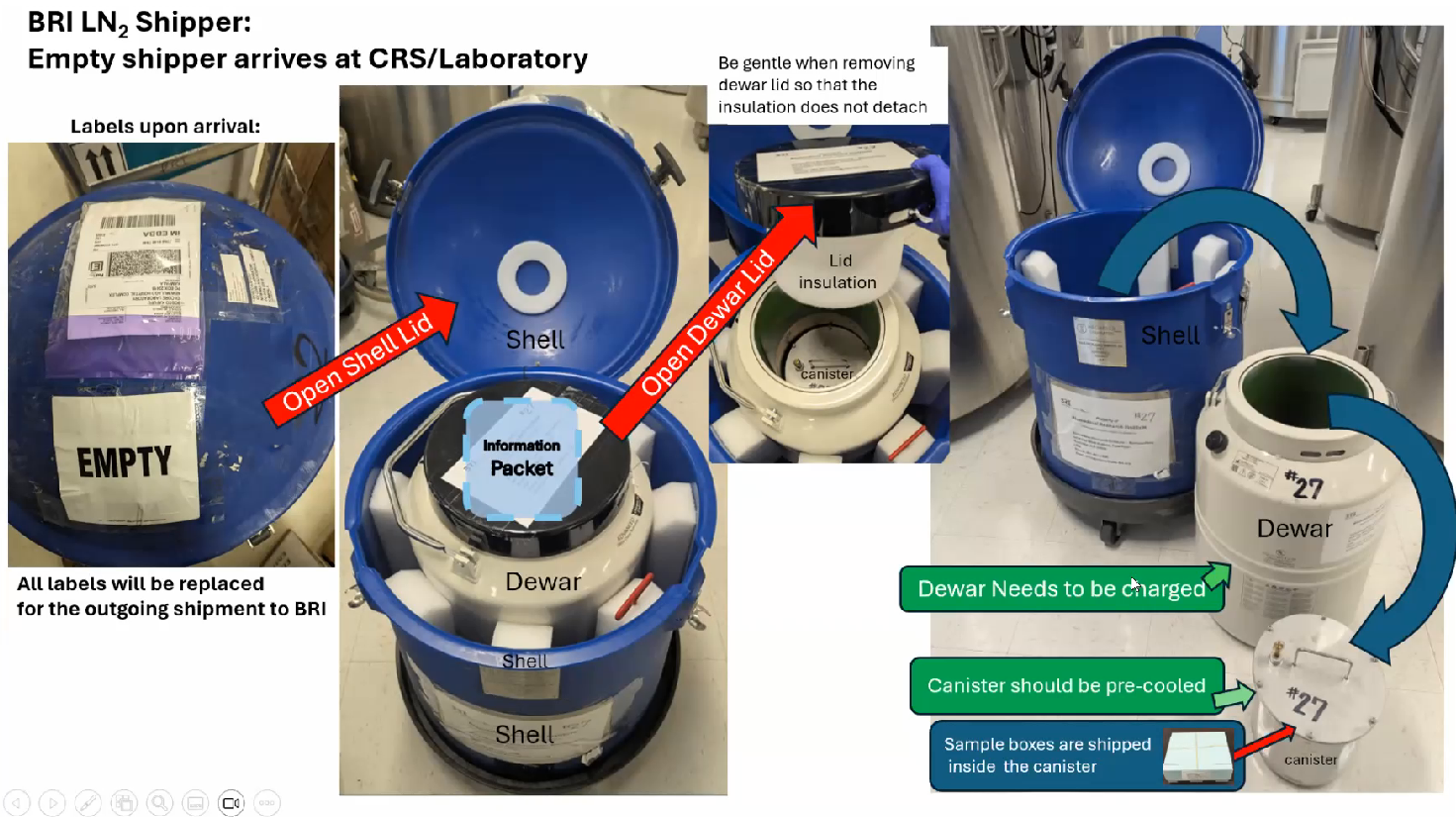
Resources
MiLab is an ACTG and IMPAACT Laboratory Center web portal that allows sites and laboratories to complete and submit documentation requirements for laboratory approvals to participate in network studies. The topics covered in this video tutorial include an introduction to MiLab, gaining access to MiLab, and navigating the MiLab homepage.
To assist sites in tracking the status of MiPALs, site users have access to the current list of all their supporting laboratories and the MiPAL information for each study in which the site is participating within their MiLab user accounts. This video tutorial covers the features of a site MiLab user account.
One key feature of the site user account is the ability to request new lab users. This video tutorial provides an overview of the process that site users follow to request a new MiLab user.
The Domestic Analyte List (or DAL) is the list of the site’s laboratories in the United States that have College of American Pathologists (CAP), Clinical Laboratory Improvement Amendments (CLIA), or equivalent certifications, and will be used to perform all the study-required testing. This video tutorial covers how to load, complete, and submit a DAL within MiLab.
Completion of the electronic Protocol Analyte List (or MiPAL) is one of the first steps and main activities that is required as part of a site’s laboratory approval process. This video tutorial provides an introduction to the concept and purpose of a MiPAL.
Site MiLab users and any affiliated laboratories receive an email notification when a MiPAL is available to be completed within the MiLab portal. The topics covered in this video tutorial include loading a MiPAL, required information for a MiPAL, and completing a MiPAL within MiLab.
One important component to understand about completing MiPALs is that labs sometimes use manual methods for analytes in which instruments do not apply. This video tutorial covers how to mark that a manual method will be used for an analyte in MiLab.
Once a MiPAL is completed and ready to be submitted for review and approval, the user submits the MiPAL to the Laboratory Center within MiLab. The topics covered in this video tutorial include how to submit a MiPAL to the Laboratory Center and the next steps after a MiPAL is submitted.
MiLab users may need to add new instruments and/or methods to their inventory list. This video tutorial covers how to add a new instrument or method to the inventory list within MiLab.
One of the DAIDS Good Clinical Laboratory Practice (GCLP) requirements for all laboratories is the documentation of appropriate validation or verification of all testing performed in support of network studies. This video tutorial provides an overview of the validation process, validation document preparation, and how to submit validation or verification documents for review within MiLab.
During the laboratory activation process, MiLab users may be asked by the Laboratory Center to provide supporting documents that verify compliance with certain elements of DAIDS Good Clinical Laboratory Practice (GCLP). This video tutorial covers how to upload and access supporting lab documents within MiLab.